1.1
Life Components ( Biochemical Important
Molecules)
The chemical molecules present in the cell and which have direct
relationship with the activities of cell
are called biochemical molecules or biomolucules. Biomolucule can also be
defined as the chemical compound found naturally in living organisms. The
collection of molecules in a cell is called cellular pool. A cellular pool
consists of:
·
Organic Compounds: Carbohydrates, Lipids,
Proteins, Amino Acids and Nucleic acids.
·
Inorganic Compounds: Minerals and water.
Inorganic compounds: It includes minerals and water.
1.2
Organic Compounds
A chemical compound containing carbon is called organic compound. Methane
is one of the simplest organic compound. Biomolecules consist primarily of
carbon and hydrogen along with oxygen, nitrogen, phosphorus and sulpher. These
elements (C,H.O and N) join in various combinations and form several types of
organic compounds such as carbohydrates, proteins, enzymes, lipids and nucleic
acids.
The organic molecules are further classified into two types.
A)
Micro molecules: They are small and simple
molecules with low molecular weight. They are formed by basic elements like
carbon, hydrogen, oxygen and nitrogen. Examples Monosaccharides, Disaccharides,
fatty acids, amino acids, nucleotides, lipids.
B)
Macromolecules: They are large and complex
molecules with high molecular weight. They are formed by polymerization of
large number of micromolecules. Example: polysaccharides, proteins, nucleic acids.
1.2.1
Carbohydrates: These are the organic compounds
of carbon,hydrogen and oxygen where the hydrogen and oxygen are present in the
ratio of 2:1, as in water. They act as sources of energy for cells. The
carbohydrates are classified as monosaccharides, disaccharides and
polysaccharides. They are called ‘Saccharides’ (Sakcharon in Greek meaning
‘Sugar’.
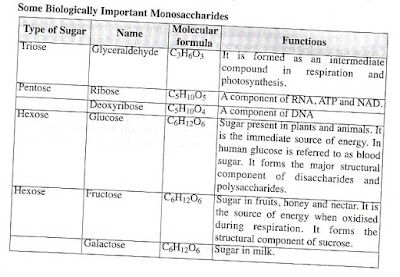
Monossaccharides: Monosaccharides are the simplest form of
carbohydrates with only one simple sugar. The general formula for
momosccharides is CHO. Based on the number of carbon atoms, the members of
monosccharides are known as trioses (C3H6O3) , tetroses (C4H8O4), pentoses
(C5H10O5), hexoses (C6H12O6) and heptoses (C7H14O7), Glucose, fructose and
galactose are the most common types of hexoses. They are highly soluble in
water and sweet in taste.
They are also designated as aldoses and ketoses depending on
whether they containaldehyde or ketone groups. All sugars contain the C=O
group. This is called a carbonyl group. When carbonyl group is terminal in
position and is jointed to at least one hydrogen atom it is designated as
aldehyde. But when it is subterminal in position or it is present between
carbon atoms it is designated as ketones.
Some Biologically Important Monosaccharides
Disaccharides: These are formed by two molecules of the
monosaccharides with release of a water molecule. The most common disaccharides
are maltose sucrose and lactose. They are soluble in water and sweet in taste.
Sucrose is found in the juice of plants such as sugarcane,
sugar beet, pineapple and carrot roots. Lactose or milk sugar occurs about 5%
in cows milk and 7% in human milk,
Some biologically important disaccharides
Polysaccharides: Polysaccharides are the condensation
product of a large number of monosaccharides. The general formula for
polysaccharide is (C6H10O5) n. Shorter polysaccharides with 2-10 monomeres, are
called oligosaccharides. Polysaccharides are mostly insoluble in water and not
sweet in taste. The most common polysaccharides found in living organisms are
glycogen, starch and cellulose. Starch is the storage food of plant . Glycogen
is the storage food of animal and also found in blue green algae, fungi and
bacteria. Cellulose is an important constituent of plant cell wall.
Cellulose is the main constituent of paper and cloth. It is
also the basis for the manufacture of several synthesis fibres like rayon.
1.1.1
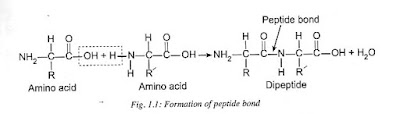
Proteins: Proteins are nitrogenous
macromolecules of amino acids. The amino acids in protein are joined together
by the peptide bonds between the carboxyl and amino groups. Hence, the proteins
are also known as polypeptides. A covalent bond between a nitrogen atom and a
carbon atom is called peptide bond. Most proteins are polymers of twenty
different amino acids.
Every cell contains protein. It is a major
part of the skin, muscles, organs and glands.
Proteins are classified into three types:
a)
Simple proteins: These proteins are made up of
amino acids only eg. Albumin, globulin, protamines, histones, glutelines, etc.
b)
Conjugate Proteins: These are the proteins which
contain other substance known as prosthetic groups in addition to amino acids.
Conjugate proteins are phosphoprotein, nucleoprotein, glycoprotein,
chromoprotein, haemoglobin etc.
c)
Derived proteins: They are products of
denaturation or of partial digestion of proteins eg. Metaproteins, proteoses
and peptones.
Functions of proteins
I)
Protein is the structural component of
protoplasm and is thus an important consitituent of all cells and tissues.
II)
All enzymes are derived from proteins.
III)
Protein also forms a part of membrane.
IV)
Chlorophyll consists of magnesium and proteins,
while the cytochromes are made up of iron and proteins.
V)
Protein is important for growth and development
during childhood, adolescence and pregnancy.
1.1.1
Amino Acids: Amino acids are small molecules of
carbon, hydrogen, oxygen and nitrogen. Sometimes, they may contain sulphur
also. They are the building blocks of protein. About 20 naturally occurring
amino acids are known. These are colourless, crystalline solids. They are
generally soluble in water and insoluble in organic solvents. An amino acid
contains one amino group (-NH2), Carboxylic group (- COOH) and R- group having
variable length of atomic grouping. The R group in glycine is a hydrogen atom,
CH3 in alanine, CH2OH- in serine: CH3CHOH in threonine.
Function of Amino acids:
a)
They are the building blocks of proteins.
b)
Amino acids are converted to vitamins, hormones,
pigments and alkaloids.
c)
Carbon chain of many amino acids is converted
into glucose after removal of amino group.
d)
When carboxyl group of amino acid is lost as
carbon-dioxide biologically active amines (histamine) are formed.
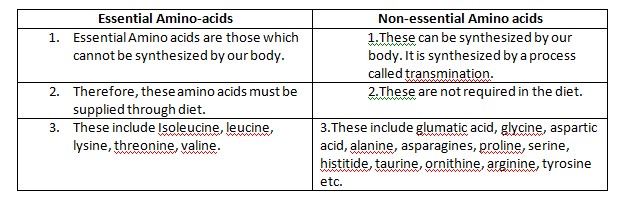
Depending upon the ability of our body to
synthesis them, amino acids can be divided into two types:
i)
Essential and ii) non essential amino acids. The
term essential or non essential does not mean that some are essential to our
body and others are non essential.
1.1.1
Lipids : Lipids are organic compounds of carbon,
hydrogen and oxygen, but have much less oxygen in comparison to carbohydrates.
Lipids are esters of fatty acids and alcohol. They are insoluble in water but
readily dissolve in organic solvents, such as ether. Benzene and chloroform .
Esters : Ester is an organic compound formed by a carboxylic
acid and an alcohol molecule with the loss of a water molecule.
Fatty Acids: These are large molecules containing the
carboxylic group- COOH.
Fatty acid molecules in living organisms commonly have up to
20 carbon atoms. Palmitic acid for example, has 16 carbon atoms.
Glycerol : It is a trihydric alcohol and can react with
three molecues of fatty acids to form a fat molecule.
Lipids are Classified into three types:
1)
Simple lipids
2) Compound lipids 3) Derived
lipids
1)
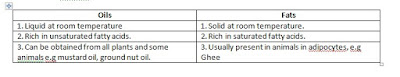
Simple lipids: Simple lipids are esters of fatty
acids and various alcohols eg.
a)
Fats : esters of fatty acids and glycerol that
are solid at room temperature.
b)
Oils : esters of fatty acids and glycerol that
are liquid at room temperature
c)
Waxes : esters of fatty acids and alcohols other
than glycerol.
2)
Compound Lipids : These are lipids, which
contain an inorganic or organic group in addition to fatty acids and alcohol.
a)
Glycolipids : Carbohydrate+ lipid
b)
Phospholipids or phosphatides : Phosphoric acid
+ lipid
c)
Lipoprotien : Protein + lipid
3)
Derived Lipids: They are derived from simple or
compound lipids on hydrolysis.
a)
Fatty acids of various series.
b)
Setrols : (steroids) : A steroid is a derived
lipid formed of four carbon rings with an attached
c)
R group. Cholesterol , ergosterol, diosgenin, stigmasterol, sitosterol
are some important sterols.
Cholesterol:
Since cholesterol was first isolated from gallstones. It was called cholesterol
which means “ Solid alcohol from bile “. Brain, nervous tissues, adrenal glands
and egg yolk are rich sources of cholesterol.
Importance
of Steroids
1)
Cholesterol is a molecule of many sex hormones
like testosterone, progesterone, etc.
2)
Cholesterol forms vitamin D on radiation by UV
rays.
3)
Diosgenin is used in the manufacture of anti-
fertility pills.
4)
Cholesterol creates abnormal thickening of the
walls of arteries. It can raise the blood pressure and hence may lead to
circulatory problems.
5)
It is not bad not useful as well.
Differences Between Fats and Oils
Functions Of lipids
i.
Fats are abundantly found in seeds as reserve
food with a large amount of energy stored in them.
ii.
They (fats) are reserve food in plants and
animals
iii.
They form an insulating layer below the skin in
animals
iv.
Phospholipids are important constituents of cell
membranes and subcellular structures
v.
They act as cushions to absorb mechanical impact
around organs like eyeballs
vi.
Lipoprotien can prevent bacterial diseases
vii.
Bile salts are modified cholesterol needed for
digestion of fat
viii.
Wax provides a protective and impermeable lining
to edidermis and check water loss in some xerophytic plants
1.2.5 Nucleic Acids: Nucleic acids are long chain macromolecules
of nucleotides . Nucleic acids were so named because they were first found in
the nucleus of cells but later they are found existing outside the nucleus.
Nucliec acid was first isolated in 1869 by a Swiss physician
F Miescher from the nuclei of pus cells. He called it nuclein. Altman(1889)
renamed it as nucleic acid. Oswald Avery (1944) gave some evidence that nucleic
acid is the carrier of genetic information.
Living organisms contain two types of nucleic acids in the
form of DNA and RNA. These are long chaims macromolecules of nucleotides with
high molecular weight. For example E coli has DNA molecule of 3,400,000 base
pairs. In higher organisms the amount of DNA may be hundred times larger (7000
times in the case of man)
Deoxyribonucliec Acid: DNA is mainly found in the nucles of
eukaryotic cell a small amount is also found in mitochondria and chloroplasts.
It is also found in the cytoplasm of prokaryotic cell. It is formed by the end
to end polymerization of a large number of repeated units called
deoxyribonucleotides or simply nucleotides. Each nucleotide is formed by cross
linking of three substances.
i.
Deoxy-ribose-sugar: The DNA molecule contains
deoxy-ribose-sugar and hence it is called deoxyribonucleic acid. Deoxyribose is
a pentose sugar (with 5 carbon atoms)
ii.
Phosphate: The phosphate in the DNA is present
as phosphoric acid (H3PO4). Each phosphate group is joined to a carbon atom 3
of one deoxyribose sugar and to carbon atom 5 of another deoxyribose sugar.
iii.
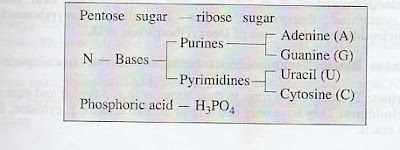
Nitrogenous Bases: The nitrogenous bases are of
two types- purine and pyrimidine . Purine bases of DNA comprise mainly adenine
(A) and guanine (G) while pyrimidine bases comprise cytosine (C) and
thymine(T).
Nucleosides and nucleotides : A sugar molecule with the
nitrogenous base forms a nucleoside and a nucleoside with a phospheat group
forms a nucleotide . The nucleosides in DNA are called deoxyribonucleosides and
nucleotides are called deoxyribonucleotide.
Nitrogenous base+sugar = Nucleoside
Nucleoside+Phosphate+Nucleotide
Or
Nitrogenous base+sugar+phosphate=Nucleotide
ADNA Molecule is composed of:
DNA is a Double Helix or Double stranded structure.
Watson and Crick established the structure of the DNA
molecule in 1953 on the basis of the x-ray diffraction. For this excellent
discovery Watson and crick were awarded by Noble prize in 1962. According to
them DNA is composed of two polynucleotide strands that form a double helix
around the central axis. These two strands run in opposite directions to each
other and are therefore, anti parallel.
The strands are made up of alternate bands of deoxyribose
sugar and phosphate molecules.
They are joined by the phosphodiester linkages. Abond between two sugar groups and a phosphate group is called phosphodiester bond.
They are joined by the phosphodiester linkages. Abond between two sugar groups and a phosphate group is called phosphodiester bond.
Each deoxyribose sugarin the
strand has one N-base horizontally attached to it at carbon-1. The fourN-bases
can occur in any possible sequence along the length og a strand. The N-base
+deoxyribose sugar+ phosphate together form one unit or each other in a linear
fashion, therefore, the resulting strand is described as the polynucleotide
strand and DNA molecule as polynucleotide molecule.
The two polynucleotide strands are held together by hydrogen
bonds between specific pairs of purines and pyrimidines. Purine of one polynucleotide
chain pairs with pyrimidine of the other i.e adenine (A) with thymine (T) and
guanine (G) with cytosine (C). A and T are held together by two hydrogen bonds
and G and C by three bonds e.g (A=T) and (G=C). The sequence of bass in one
polynucleotide chin automatically determines the order of bases in the other
i.e the two chains are complementary to each other. For example, when adenine
(a purine) occurs in one strand , thymine ( a pyrimidine) is present in the
corresponding position in the opposite strand and vice versa. Similarly
wherever guanine (a purine) is present in one strand, the other strand has cytosine ( a
pyramidine)opposite to it and vice versa.
The two strands of a helix are of opposite polarity. If one
chain runs in 3-5 direction (sugar phosphate linkage) then the other will run
in 5-3 direction (sugar phosphate linkage).
Circular DNA molecule
Circular DNA molecule are found in almost all prokaryotes
e.g bacteria. The molecule has two complementary strands which form a covalenty
closed circular DNA. This DNA is supercoiled and highly folded. This is because
the diameter of a bacterial cell (E coli) is about 1-2 microns, while the total
length of the circular DNA is about 1100 microns. In several groups of small
bacteria and viruses the circular DNA is single stranded. It becomes double
stranded only during replication.
Functions
I.
DNA is a genetic material hence it carries all
the hereditary information from one generation to another generation.
II.
DNA has unique properties of formation of carbon
copies. This is essential for transfer of genetic information.
III.
DNA gives rise to RNA through transcription
process.
IV.
DNA play a key role in protein synthesis.
V.
Any change in the sequence of nitrogen bases due
to addition or deletion causes mutation.
Ribonucleic Acid (RNA)
RNA is found both in the nucleus
and in the cytoplasm . It is a single
stranded, long chain macromolecules of ribonucleotides. Like DNA the
ribonuucleotide of RNA is also formed by cross-linking of three substances-phosphoric
acid a pentose sugar (ribose sugar) and nitrogen bases.The ribose sugar differs
from the deoxyribose sugar of DNA in having four rather than three hydroxyl
groups. The nitrogenous bases of RNA are of two types: i) purine ii) pyrimidine
Purine bases comprise adenine A)
and guanine G) while pyrimidine bases comprise cytosine C) and Uracil U).
Thus structurally DNA and RNA
show two main differences:
i) DNA contains deoxyribose and
RNA contains ribose.
ii) DNA contains thymine whereas
the RNA contains Uracil.
A RNA molecule is composed of:
Being single stranded the
nitrogen bases of RNA unpaired and the complementary bases found in the same
strand.The four nucleotides in the RNA are A, G,C and U nucleotides. In a
double stranded RNA base pairing occurs between purines and pyrimidies.
Guanine(G) and Cytosine (C) are held together by three hydrogen bonds and
uracil (U) adenine (A) are held together by two hydrogen bonds.
The strand is made up of
alternate bands of ribose sugar and phosphate (H3PO 4) molecule . A nitrogen
base a ribose sugar and a phosphate form a nucleotide and a nucleotide without
the phosphate is called nucleoside.
In most of the plant viruses and
half of animal virus the genetic materials is RNA rather than DNA.
The RNA is classified into three types.
1.
M-RNA ( messenger RNA)
2.
R- RNA (Ribosomal RNA)
3.
T- RNA ( transfer- RNA)
1. m- RNA (messenger
RNA) : It constitutes about 5%- 10% of the total RNA present in the cell m-RNA
carries the genetic information from DNA for protein synthesis.
2. r- RNA ( Ribosomol
RNA) : It takes about 80% of the total RNA in the cell. It is the major
component of ribosomes. It also provides proper binding sites for the mRNA on
the ribosones.
3. t-RNA (Transfer RNA) It is also known as soluble RNA
(sRNA) . It forms about (10%-15%) of the total cell RNA . It carries amino acid
molecules to the site of protein synthesis.
Nucleotide
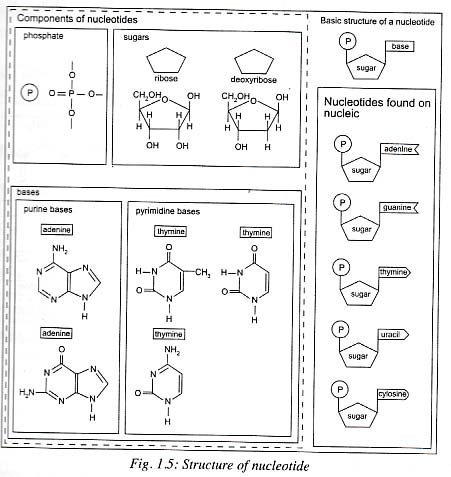
A nucleotide is made up of three molecules . These are:
A) Pentose sugar b) Nitrogenous bases and c) Phosphoric acid
A) Pentose Sugar: There are two kinds of pentose sugar found
in nucleotides. These are ribose sugar and deoxyribose sugar. A nucleotide
containing ribose sugar is called ribonucleotide. Ribonucleotide is the basic
unit of ribonucleic acid (RNA) . A nucleotide containing deoxyribose sugar is
called deoxyribonucleotide. Deoxyribonucleotide is the basic unit of
Deoxyribonucleic acid.
B) Nitrogenous Bases: The nitrogenous are of two types
purines and pyrimidines.
I.
Purines: They have two rings in their structure
eg Adenine and guanine.
II.
Pyrimidines: They have only one ring in their
structure e.g cytosine, thymine and uracil.
c) Phosphoric Acid: It contains a phosphate group. It links
two nucleotides together by information of phosphodiester bond.
Nucleosides and Nucleotides
A sugar molecule
together with the nitrogen base forms a nucleoside. These are ATP , ADP, AMNP,
MNAD, MNADP , FAD.
An ATP contains a ribose sugar the base adenine and three
phosphate groups. The energy transporting portion of ATP is a bond between the
terminal and middle phosphates. These bonds on hydrolysis yield adenosine diphosphate
(ADP) on removal of one phosphate and adenosine monophosphate (AMP) on removal of two phosphate groups. ATP
is universal carrier of energy in living cells. Major ATP synthesis takes place
in mitochondria during respiration.















0 comments:
Post a Comment